Background
Hydrogenation of furfural, a process used to convert biomass into useful chemicals, can sometimes produce unwanted byproducts like furan and THF due to breaking carbon–carbon bonds. We use computational simulations such as VASP DFT and NEB to study the reaction at the atomic level and explain why pure platinum (Pt) and platinum–tin (PtSn) alloy surfaces favor different reaction pathways, helping us understand which surface produces more of the desired product
Methods
- VASP Density Functional Theory (DFT) for relaxations of initial and final states and thermodynamic analysis
- Nudged Elastic Band (NEB) for reaction coordinates and kinetically feasible routes
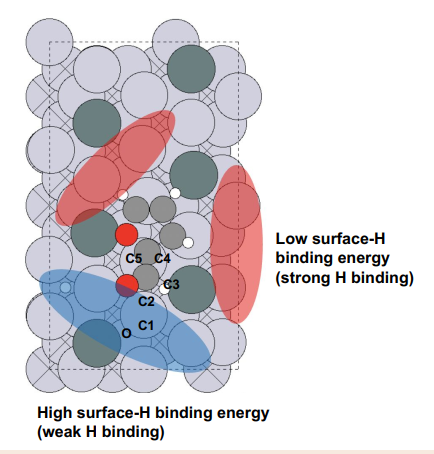
- Surface Models: Pt(111) vs PtSn with distinct H binding characteristics
Key Results
- Pt(111): strong H bindings → C–C scission routes accessible → byproducts
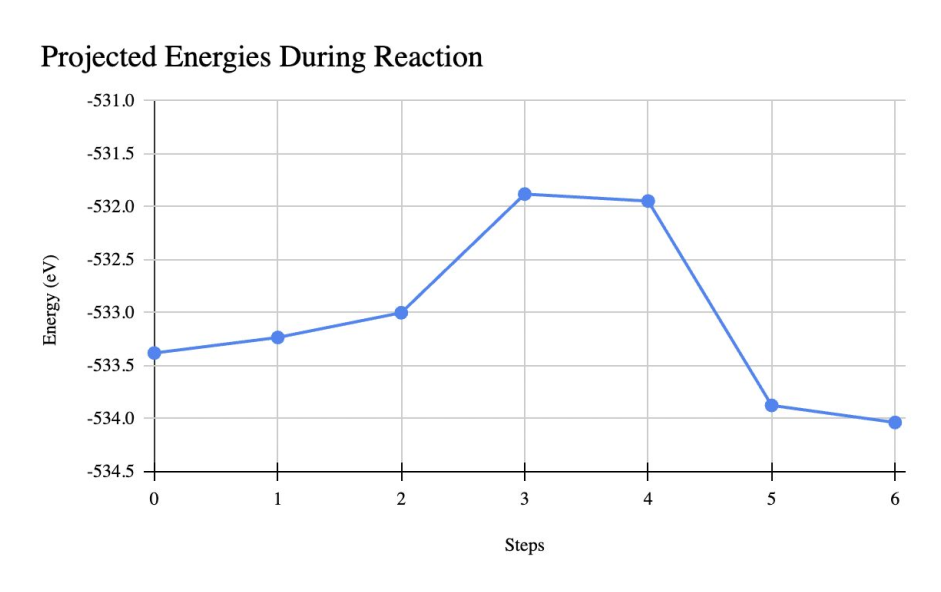
- PtSn: Sn donation weakens local H binding and raises barriers (~2 eV) for unwanted routes, while keeping carbonyl hydrogenation favorable/selective
Impact
Electronic modification by Sn explains the improved selectivity of PtSn catalysts, providing a mechanistic basis for designing alloy surfaces that minimize byproduct formation in biomass upgrading
Docs